A non-coding mutation linked to extreme obesity
A tandem duplication rewires a tissue-specific gene to a ubiquitous promoter to cause extreme obesity
Happy Friday! A few days ago, I was thinking about non-coding variants and their role in drug target discovery. One thing I am curious about is if the emerging large whole genome sequencing databases will lead to discoveries of rare non-coding variants that restrict a gene’s expression to a specific tissue or to a specific developmental time point. Such discoveries could help one current limitation of using rare coding variants for drug discovery, which is their inability to differentiate tissue specific and developmental stage-specific effects of gene perturbations. This is important because, often, good targets are mistakenly tossed out as bad ones, assuming that inhibiting them will cause serious adverse effects. A gene’s role during early development can be independent of its role in adults. I wrote about an example, DGAT2, on Twitter a few days ago. Likewise, a gene’s role in one tissue can be independent of its role in another. BCL11A is a good example for this. BCL11A haploinsufficiency causes neurodevelopmental disorder, but that is not of concern when you want to knock it down using CRISPR ex vivo only in the blood stem cells extracted out of the patient.
Identifying non-coding rare variants that imitate tissue-specific and developmental stage-specific knockdown is not entirely impossible. We have seen examples where Mendelian types of diseases caused by non-coding mutations that disrupt regulatory circuits, like HK1 intronic mutations releasing the beta cells-specific silencing of hexokinase expression resulting in hyperinsulinism and a structural variant that rewires a tissue-specific gene, ASIP, to a ubiquitous promoter resulting in extreme obesity. My Twitter story of the latter example, one of the popular ones, is my choice for this week’s post.
From the Twitter archives
Agouti gene (Asip) is the first obesity gene to be discovered in 1992 in agouti mice--a fat yellow mice with a mutation that reprograms a skin specific protein to express throughout the body. However, no similar mutations in this gene have been found in humans so far.
Thirty years since its discovery, now scientists have encountered the first human counterpart of agouti mice—a girl with red hair, hyperphagia, extreme obesity, tall stature, severe hyperinsulinemia and hepatic steatosis.
Routing screening for mutations in known obesity genes came negative. Then, the scientists looked for clues in the adipose tissue biopsied from the girl during the gastric bypass surgery (that helped the girl to lose 40kg weight !!) and were awestruck at the result: a single gene, the very gene that causes obesity in agouti mice, was expressed several hundred folds compared to expression in control tissues.
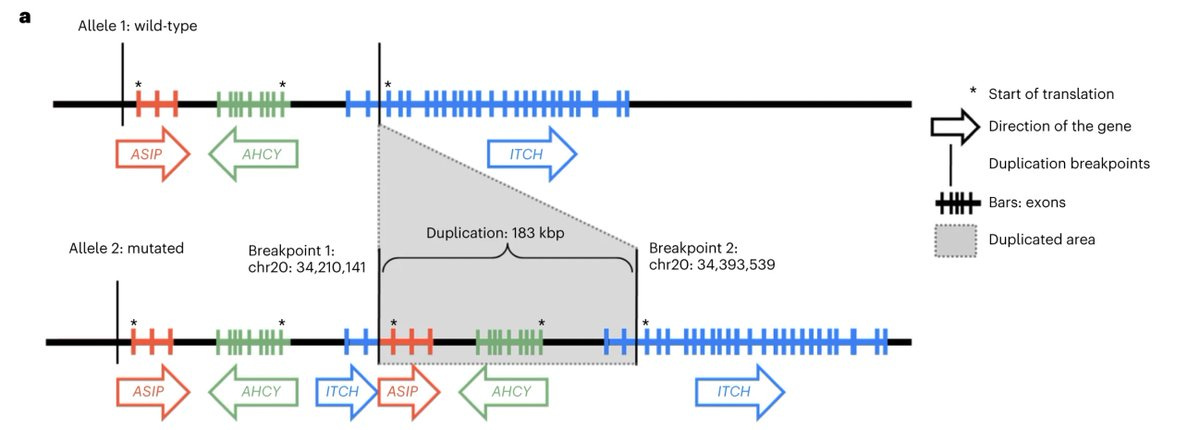
Going back to the girl's WGS data and looking closer at the locus of this super-expressed gene, the scientist could see now clearly what is the cause: a tandem duplication that not only duplicated the ASIP gene but also switched its promoter with that of a nearby gene, ITCH.
ASIP is wired to be expressed only in skin, while ITCH, throughout the body, But in this unfortunate girl, ASIP and ITCH promoters are cross wired and so, ASIP is transcribing multiple copies in every cell of her body, including the appetite-regulating neurons in the brain hypothalamus.
ASIP is an antagonist of MC1R and MC4R. It binds to MC1R in the skin and hair follicles to stimulate secretion of pheomelanin leading to light skin, red hair and freckles. In the hypothalamus, ASIP binds to MC4R and increases appetite.
Inspired by the discovery, the scientists searched through genetic data of 1,745 individuals from the Leipzig childhood obesity cohort and found four (3 girls and 1 boy) with the exact same mutation. All the three girls had similar phenotypes: hyperphagia, red hair and increased ASIP expression in blood cells. But the boy did not have any of these. But instead he had intellectual disability. (It's not clear if there's a sex specific factor).
And the good news about this discovery is there exists already an FDA approved medicine that could be used for this condition: setmelanotide, a MC4R agonist that might be able to reverse the ASIP's antagonism on MC4R in the brain.
The whole story is absolutely mind-blowing. It is stories like this why I love human genetics so much.