Let's talk about CNVs
A micro-review on the origins, (epi)genetics and therapeutics of Angelman syndrome
Happy Friday! This week’s pick for From the Twitter archives is a post I wrote last year on the fascinating history of Angelman Syndrome after stumbling upon a great CNV paper. Enjoy!
From the Twitter archives
Great paper on the genome-wide associations of copy number variations (CNVs) with seizure disorders. If I have to choose one thing that is most fascinating of all human genetic variations, it is CNV. Let’s talk about CNVs.
Copy number variations are large structural alterations of the human genome that increase or—often—decrease the gene copies. CNVs frequently fracture large fragments of the human genome, and expectedly, show high penetrance. So, there is no shortage of phenotypes. The major challenge though is to map which gene is causing which phenotype.
In the past few days, we have seen stories of GWAS loci that have been hammered by scientists for decades to reveal their secrets. If you were amazed by those stories, wait until you hear about CNVs that have been hammered for more than a century and they haven’t spilt even half of their secrets yet.
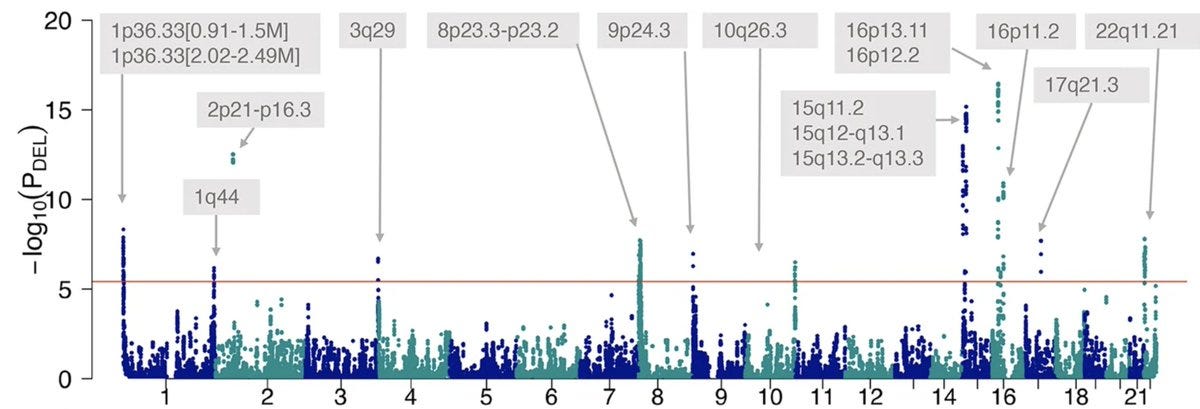
Let’s zoom into one of the tallest towers, rising from chromosome 15, of this Manhattan plot, which holds a fascinating puzzle—15q11-q13 deletions—that evolution has handed over to human geneticists to solve.
Origins of Prader-Willi and Angelman syndromes
The earliest record of Prader-Willi syndrome dates back to the 1880s. John Langdon Down (famously known for his discovery of Down syndrome) in his classical text Mental Affections of Childhood and Youth described a case of 14 yr old, short-statured, obese and intellectually disabled girl. But these facts only came to light long after the three Swiss doctors Andrea Prader, Heinrich Willi and Alexis Labhart described in 1956 a case series of nine children with the tetrad of short stature, obesity, intellectual disability and small hands and feet and birthed the name “Prader-Labhart-Willi syndrome”. Over time, the syndrome lost its middle name for some reason and now it is today just “Prader-Wili Syndrome”.
The earliest record of Angelman Syndrome dates back to 1965 when an English physician described a case series of three children that he likened to “happy puppets” because of their phenotypes—laughing demeanour and jerky gait.
Needless to say, it took many decades since the first case reports for genetic technologies to advance to know that these syndromes are caused by deletions in the chromosome 15q11-q13 region.
Prader-Willi and Angelman—the poster children of imprinting disorders
What makes these syndromes fascinating is not merely their structural alterations but the maternal and paternal biases in the expression of those structural alterations. Angelman occurs due to deletions of the 15q11-q13 region that is expressed only in the maternally inherited segments (Angel: Mother, a mnemonic from med school days that is still alive in my memory) and in Prader-Willi, it’s the other way around.
Decoding the evolutionary designs behind imprinting
One of the Prader-Willi and Angelman puzzles that scientists have been slowly piecing together for decades is understanding the master design that went into the unique maternal and paternal imprinting of the 15q11-q13 regions. The imprinting design of Angelman is my favourite. Many of the clinical features of Angelman Syndrome are due to the deletion of an extremely critical gene UBE3A which codes for a ubiquitin ligase and is the master janitor of our cellular buildings; it keeps our cells tidy by degrading the damaged and discarded proteins. And you can imagine the protein chaos that will unfold when this janitor quits.
The earliest clues surfaced in 1998 when scientists were poking around the brains of dead people with Angelman Syndrome. They spotted something unusual in the gel electrophoresis pictures that displayed the UBE3A transcripts amplified using RT-PCR. One band, the expected one, corresponded to spliced UBE3A mRNA. But what is the other one? Scientists realized that it was an unspliced UBE3A transcript that came from the paternal allele (unlike the main UBE3A which, as expected, came from the maternal allele). Interestingly, that transcript was amplified only by the primer that recognized the UBE3A sequence oriented inversely (anti-sense).
Everything fell into place then. It was a naturally occurring UBE3A anti-sense transcript that evolution has placed in the paternal line to ensure that paternal UBE3A is not expressed in the neurons (UBE3A is imprinted only in neurons).
Therapeutic exploitation of an evolutionary design
How do you re-express the UBE3A in Angelman patients who lost their only active copy? Reverse engineer the evolutionary design. Silence the UBE3A antisense transcript which will release the imprinting in the paternal chromosome resulting in UBE3A expression. The design works in mice. Will it work in humans? We will know soon in the coming years.
The challenge of mapping genotypes to phenotypes
Just a few days ago, we saw how scientists were struggling to identify causal genes at the FTO GWAS locus. Imagine a similar scenario, but this time instead of one or two causal genes, we have dozens, each of which has its own set of phenotypes and all massively overlap in patients. Compared to the GWAS locus, dissecting the causalities in a CNV locus is many folds complicated. Scientists use many approaches to map the genes in the CNV regions to their phenotypes. One by studying naturally occurring loss of function and gain of function mutations in individual genes and overlapping their phenotypes with CNV phenotypes. This approach easily exposes the genes that have a typical mirror effect.
A beautiful example is the gene MMP23, one of the affected genes in CNV 1p36 (the tallest tower in chr 1 in the Manhattan plot). MMP23 codes for a matrix metalloproteinase and controls tissue and bone remodelling. Loss of MMP23 delays cranial bone fusions resulting in large anterior fontanelles in the affected babies and gain of MMP23 fuses cranial bones too early resulting in craniosynostosis.
But not all genes are as easy as MMP23 to understand. In fact, MMP23 is the only gene that was confidently mapped to a phenotype in the 1p36 locus. I recently discussed another similar example: mapping the OCA2 in the 15q11-q13 region to skin pigmentation.
CNVs are some of the best examples to showcase the challenges of genotype to phenotype mapping in human genetics, which takes collective efforts that often span many many decades and hundreds of scientists. Congrats to Dennis Lal and the team for pushing such efforts one step forward in the right direction.





Small science world. Dennis' lab was right next to the one I did my PhD in!