MSH3, a promising drug target for Huntington's disease
A new study reports that knocking down MSH3 halts the CAG repeat expansion in Huntington's disease
Today, I stumbled upon an interesting Scientific Reports article that reports that knocking down the DNA mismatch repair gene MSH3 in Huntington’s mice model dose-dependently reduced the somatic expansion HTT CAG repeats.
Drug development for Huntington’s is a topic that I started following closely only recently. For some reason, I never read much on this topic except for occasional GWAS studies. I knew the basics about the disease and a little about some of the inspiring fieldwork by scientists like Nancy Wexler in Venezuela that led to the groundbreaking discovery of Huntington’s gene. But otherwise, for most of my career, I was oblivious to the fascinating stories that have been unfolding for past decades in the Huntington’s field.
Recently I’ve been hearing about Huntington’s drug development a lot in news and articles which nudged me to start reading about it. Perhaps, this STAT article is what mainly opened the window for me into the world of Huntington’s drug development (I strongly recommend reading it). Also, one of the talks on Huntington’s at last year’s American Society of Human Genetics (ASHG) conference blew my mind and induced more curiosity in me to learn more about this topic. I spoke a little bit about it in the year-end special episode of The Genetics Podcast by Patrick Short from Sano Genetics.
Hopefully, I’ll find time in the future to write a deep dive article for GWAS stories, focussing on the human genetic findings and their impact on Huntington’s drug development. But for now, I wanted to highlight to you this article that I bumped into today and perhaps nudge some of you into digging into Huntington’s literature.
The role of DNA mismatch repair genes in modifying HTT repeat expansions has been long known for more than 20 years now, through both mice and human studies. But the interest in targeting DNA repair genes to treat Huntington's took off after GWAS studies in Huntington patients implicated MSH3 specifically. There are also interesting debates about the causal variant at the MSH3 locus, which was initially thought to be a missense variant and then found to be a repeat variant that decreases MSH3 expression (a repeat mutation in one gene is modifying the pathogenicity of a repeat mutation in another gene, fascinating, isn’t it?).
A more interesting fact is that the mechanism of MSH3's action on repeat expansion is likely disease-agnostic; reducing its expression likely protects the expansion of other repeats as well, for example, CTG repeats in myotonic dystrophy type 1.
The mechanism of how MMR genes expand the HTT repeats in the striatal neurons is still not clear. Scientists believe that the MMR genes try to fix the DNA loops (that form due to polymerase slippage), which they see as a repair in the DNA. But instead of fixing it, they make it worse by adding more repeats. Although the mechanism is not clear, there is overwhelming evidence that blocking MSH3 will prevent somatic expansions, which has been shown experimentally in mouse models.
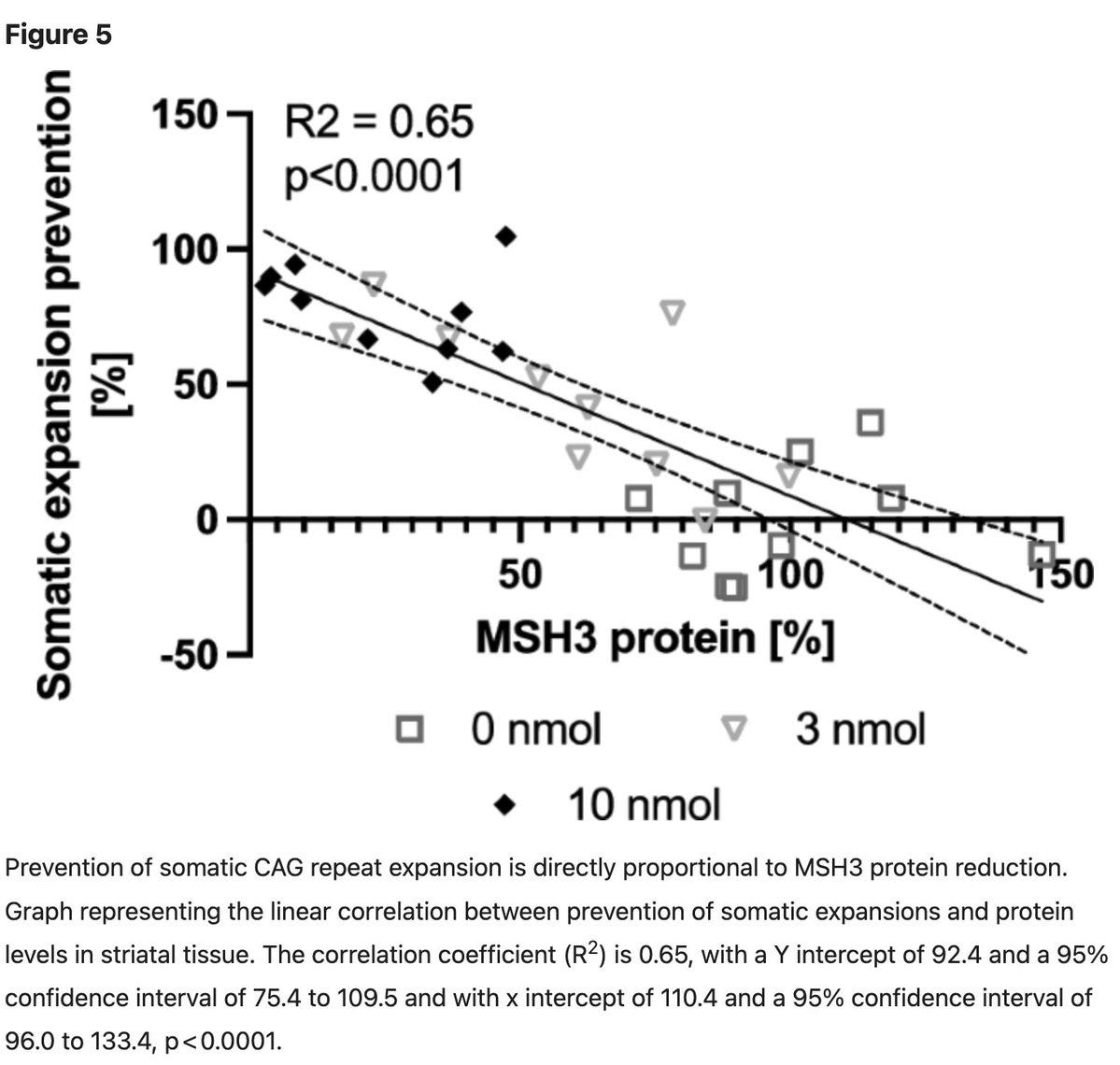
In the current paper, the authors replicate that knocking down MSH3 in the HTT mice model halts repeat expansion. But then, they also add some additional insights. They find that the MSH3 knockdown doesn't have any effect on the repeats that were already expanded. It only prevents future expansion, hence highlighting the importance of the timing of intervention. This point will hit you hard when you learn about the revised model of Huntington’s disease presented at the ASHG last year by Bob Handsaker from Steve McCarroll’s group at the Broad Institute. The timing of intervention is the most critical part of treating Huntington’s and according to the revised model, there might be a therapeutic window before the repeat expansion clock starts ticking fast that can be exploited to halt the disease progression.
Then, the authors also show that there is a one-to-one relationship between MSH3 knockdown and the prevention of repeat expansion. That is, to prevent repeat expansion by 100%, one will need to knock down the MSH3 by 100%, which is impossible and may not be safe as, you know, MSH3 is a cancer gene. Though desired therapeutic efficacy is still not clear, even a 50% knockdown that appears to be safe in humans (informed by human genetics) might have a meaningful impact on disease progression.
The good news is many academic and industry researchers are actively working on this problem, and more exciting studies will likely emerge in the near future. When that happens, you’ll hear all about it in the GWAS stories.