Looking back at a failed drug pipeline through the lens of human genetics
The rise and fall of DGAT1 inhibitors as obesity and type 2 diabetes drugs.
Happy Friday! A few days ago, I was searching for examples of drug targets whose failures in human trials due to adverse effects could have been predicted or at least, were retrospectively realized using human genetics. One example that always surfaces first in my memory is the DGAT1. I learned about this example from a review article on pharmacogenomics by Munir Pirmohamed, which I summarized in a Twitter thread last year. Relating closely to the theme of last Friday’s post (using human genetics to ensure the safety of a drug target), the thread on the rise and fall of DGAT1 inhibitors for the treatment of obesity and type 2 diabetes, I felt, is a good choice for this week’s From the Twitter archives post. If you happen to know any such examples, please let me know in the comments.
From the Twitter archives
I am always on the lookout for examples of human genetics informing drug adverse effects. Here is an interesting one I recently came across in this great review article on the past, present and future of pharmacogenomics.
Diacylglycerol acyltransferase 1 (DGAT1) inhibitor is one of the many drug targets drug companies pursued purely based on biochemistry knowledge and findings based on animal models but never saw the light of the day.

The final step of triglyceride synthesis is catalyzed by two structurally different but functionally similar enzymes: DGAT1 and DGAT2. The two enzymes account for nearly all the triglyceride synthesis in the body. The loss of one is likely compensated by the other.
Understandably, drug developers found the DGAT enzymes as attractive drug targets to treat obesity and type 2 diabetes. Scientists started exploring the consequence of partially or completely deleting DGAT1 or DGAT2 in animal models.
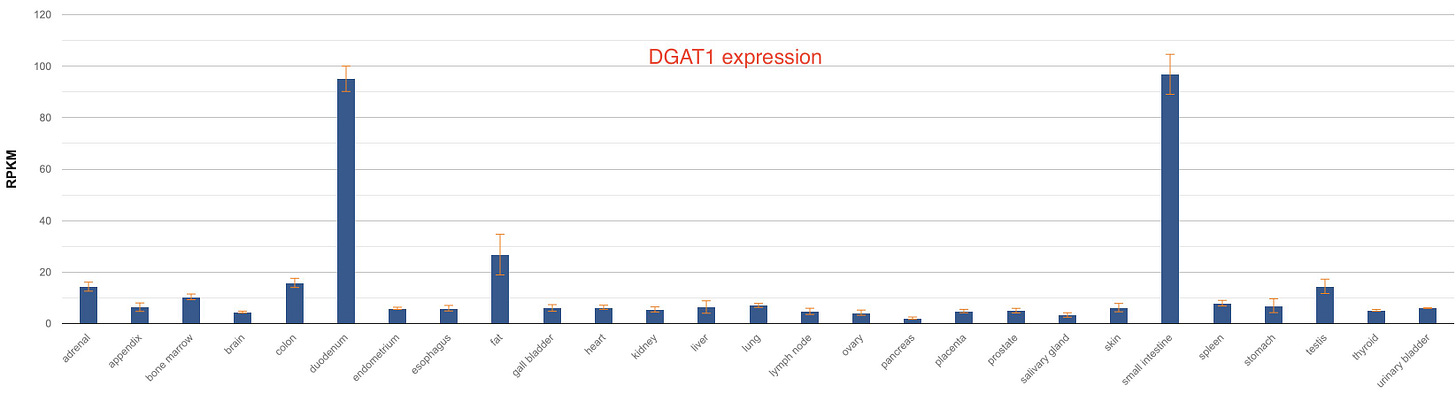
There are some interesting differences in the tissue distribution of these two enzymes in humans. While DGAT1 is expressed predominantly in the intestine and fat tissues, DGAT2 is expressed highly in the liver, fat and skin.
Animal experiments revealed that DGAT2 deletion is lethal. The mice lacking DGAT2 died soon after birth from dehydration caused by rapid water loss through their leaky skin. So, that ruled out DGAT2 as a drug target1.
On the other hand, the scientists found that the DGAT1 knockout mice were viable, metabolically healthy and lived longer than the wild-type mice and importantly, showed impressive resistance to diet-induced obesity and glucose intolerance.
This led to the development of DGAT1 inhibitors. In 2012, AstraZeneca (AZ) scientists published the discovery of a small molecule AZD7687, a potent and selective inhibitor of DGAT1.

In 2014, the AZ scientists reported the results of the first human trial. A single dose of AZD7687 resulted in a dramatic reduction in postprandial triglycerides rise. But there was a problem: the participants had severe gastrointestinal side effects. The authors concluded that the attenuating effect of AZD7687 on postprandial TAG excursion provides proof of mechanism with respect to gut DGAT1 inhibition. However, dose and diet-related gastrointestinal side effects may impact further development of DGAT1 inhibitors.
In 2015, the AZ scientists reported on a follow-up trial done mainly to evaluate the safety and tolerability of AZD7687. The drug was administered in multiple doses and the participants were evaluated if they tolerated GI side effects over time.
Sadly, the results weren’t as the scientists expected. With increasing doses, GI adverse effects only became worse, and more than half of the participants dropped out. As the trial concluded, the company hammered the final nail in the coffin of the DGAT1 drug program. The lack of therapeutic window owing to GI side effects of AZD7687, particularly diarrhea, makes the utility of DGAT1 inhibition as a novel treatment for diabetes and obesity questionable, the authors concluded the trial report.
The part of the DGAT1 story that interested me most is a case report on two children who were knockouts for DGAT1 published in JCI in 2012. In this paper, the authors describe two pediatric cases of congenital diarrheal disorder (CDD) born in an Ashkenazi Jewish family and were found to be complete knockouts for DGAT1.
Case 1 was a girl child born normal but developed severe vomiting, colicky pain and watery diarrhoea on the 3rd day after she was fed breast milk. She couldn't take anything orally and was put on total parenteral nutrition. Puzzlingly, the child had elevated triglycerides and cholesterol blood levels (so did her mother who had partial DGAT1 deficiency) in contrast to what was seen in the DGAT-deficient mice. Unable to tolerate the parenteral nutrition, the child succumbed to malnutrition and sepsis at 17 months of age.
Case 2 was a boy, the girl's brother, with a similar history: born normal but on 3rd day got vomiting and diarrhoea and was put under total parenteral nutrition, which, unlike his sister, he tolerated and recovered from malnutrition. Like his sister and mother, he also had elevated blood lipid levels, for which he was treated with cholestyramine. He made a full recovery and at 47 months of age, he was thriving on an unrestricted diet.
Exome sequencing of the family revealed that both the children were homozygous for a splice donor variant that skipped an entire exon resulting in a complete loss of DGAT1. The chance of observing this homozygous mutation in the general population is 1 in 50-100 million.
It's amazing to see how a rare genetic disease mirrored the adverse effects of a drug designed to treat a common disease. It's not clear though how DGAT1 loss (or inhibition) leads to diarrhoea only in humans. Unlike in humans where only DGAT1 is expressed in the intestine, in mice both DGAT1 and DGAT2 are expressed in the intestine. So, it's possible that in mice, DGAT2 in the intestine compensates for the loss of DGAT1.
DGAT1 adds to the list of genes where animal models fail to recapitulate human physiology and reminds us again of the long-known wisdom that the best animal model to study human diseases is humans themselves.
It appears that DGAT2 inhibitors are currently being pursued for NAFLD, despite the initial hesitance due to the nonviability of DGAT2 knockout mice. A quick search led me to this recent article from Pfizer scientists which reads “Conversely, DGAT2 inhibition, although initially not pursued as aggressively as a potential target for pharmacologic intervention, has consistent efficacy in nonclinical studies, with reduced triglyceride synthesis accompanied by reduced expression of genes essential for de novo lipogenesis. In addition, early clinical data indicate antisteatotic effects with DGAT2i ervogastat, in participants with NAFLD, accompanied by a well-tolerated safety profile.” This reminds me of a whole other topic of differentiating the consequences of prenatal from postnatal gene loss. That’s a topic for another day.